Blog
We are excited to announce the release of the Office of Research Integrity’s (ORI) 2024 Annual Report. This report is designed to provide information about research misconduct proceedings, assurance and compliance programs, and ORI's grant programs. Data provided by ORI during calendar year 2024 in this report include:
The Office of Research Integrity (ORI) is pleased to announce the release of new guidance documents for the updated Final Rule. The updated regulation will apply to all institutions on January 1, 2026. As we approach the applicable date of the 2024 Final Rule on Public Health Service (PHS) Policies on Research Misconduct, ORI is dedicated to guiding institutions in successfully complying with regulatory requirements. Our aims are to ensure a clear understanding of the updated regulation and to foster environments that prioritize research integrity.
The Office of Research Integrity (ORI) is pleased to announce the release of the second phase of updated Final Rule Guidance Documents. As we approach the applicable date of the 2024 Final Rule on Public Health Service Policies (PHS) on Research Misconduct, ORI is dedicated to guiding institutions in successfully complying with regulatory requirements. Our aim is to ensure a clear understanding of the updated regulation and to foster environments that prioritize research integrity.
Recordings and slide decks from the July 15, 2025 Notice of Funding Opportunity Technical Assistance Webinar is now available.
ORI released three notice of funding opportunity announcements for projects, conferences, and innovative tools that advance the evolving field of research integrity and produce tangible outcomes related to ensuring the integrity and reliability of Public Health Service funded research.
The Office of Research Integrity (ORI) is excited to announce the release of the first phase of our updated Final Rule Guidance Documents! As we work toward fostering a culture of research integrity, these documents are designed to assist PHS-funded stakeholders in understanding and implementing the 2024 Final Rule, which will apply to all institutions on January 1, 2026.
ORI is hiring! We are seeking professionals with experience supervising a team, providing oversight for research misconduct programs, advising on matters related to research misconduct investigations, and implementing policies and programs related to research integrity. The pay range is between $163,964- $191,900 (GS 15).
As a Supervisory Health Scientist Administrator, you will:
Job Opportunity – Lead Public Health Advisor (Research Integrity)
ORI is hiring! We are seeking professionals with knowledge of and experience with planning, designing, and carrying out administrative reviews and analyses to optimize business results and customer experience. The pay range is between $$139,395 - $181,216 (GS 14).
As a Lead Public Health Advisor, you will:
ORI has updated its interactive videos The Lab and The Research Clinic
The Office of Research Integrity’s goals are to advance research integrity, protect Public Health Service-funded research, and build and support research integrity communities. As part of these efforts, ORI has long supported the development of educational materials to help foster an environment of research integrity and promote the responsible conduct of research (RCR).
The U.S. Department of Health and Human Services is Updating its Policies on Research Integrity to Meet the Demands of the Modern Research Environment
September 17, 2023 | By: Sheila Garrity J.D. M.P.H. M.B.A, Director of the Office of Research Integrity, Office of the Assistant Secretary for Health, Department of Health and Human Services
Summary: ORI Releases the 2024 Public Health Service Policies on Research Misconduct
The OASH Office of Research Integrity (ORI) announces the departure of Dr. Alexander Runko, Director of the Division of Investigative Oversight (DIO). During his tenure spanning over 11 years, Dr. Runko has served with tremendous dedication to ORI and the Research Integrity Officer (RIO) community. Over that time, he worked to provide oversight and preserve research integrity for Public Health Service (PHS)-funded biomedical research across the U.S. and abroad with sensitivity, rigor, and good humor.
To ORI stakeholders, research institutions, and colleagues:
We invite you to join ORI on Friday September 20, 2024, for a webinar to present the Office of Research Integrity’s 2024 Final Rule.
- BLOG
Scientific breakthroughs from PHS-funded research save countless lives worldwide through research on cancer, vector-borne diseases, and the health effects of climate change. ORI’s mission is to preserve the integrity of these scientific advancements that improve public health in the U.S. and across the globe. As scientific research continues to evolve, ORI’s work to protect these important advancements is evolving, too.
Job Opportunity - Research Integrity Specialist
ORI is hiring! We are seeking professionals with institutional experience handling allegations of research misconduct, complying with federal research regulations, and implementing policies and procedures related to research integrity. The pay range is between $82,764 - $153,354 (GS 11-13).
As a Research Integrity Specialist, you will:
The Office of Research Integrity (ORI) congratulates Dr. Karen Wehner for her selection as the Scientific Integrity Officer for the U.S. Department of Health and Human Services. Dr. Wehner has served as the Director of the Division of Education and Integrity at ORI since March 2020. On August 12, she will join the Office of the Assistant Secretary for Planning and Evaluation (ASPE) Office of Science and Data Policy (SDP) in her new role.
ORI has released its Annual Report for FY 2023. The report highlights ORI’s activities from the past fiscal year in the following areas:
- Investigative Oversight
- Communications with Community Members
- Intramural Projects
- Grants
- Compliance
The report is available in digital (PDF) format and can be downloaded by clicking on the file below.
ORI’s Division of Education and Integrity (DEI) will be hiring a Lead Public Health Advisor (Research Integrity) at the GS 14 level (pay range is $139,395 - $181,216).
As a Lead Public Health Advisor, you will use your knowledge of and experience with planning, designing, and carrying out administrative reviews and analyses to optimize business results and customer experience by:
ORI’s Division of Investigative Oversight (DIO) will be hiring a Supervisory Health Scientist Administrator (Investigations) at the GS 14 level (pay range is $139,395 - $181,216) to serve as the DIO Deputy Director. This opportunity is open to current HHS Office of the Assistant Secretary for Health (OASH) Federal career or career conditional employees only. A Bachelor's or graduate/higher level degree in an academic field related to the medical field, health sciences, or allied sciences, appropriate to the work of the position, is required (transcripts are required).
Just over a year ago, Sheila Garrity took the job as director of ORI to continue the agency’s mission:
ORI’s Division of Investigative Oversight (DIO) will be hiring Health Scientists at the GS 12-13 levels (pay range is $99,200 - $ 153,354) and Health Scientist Administrators at the GS 14 level (pay range is $139,395 - $181,216). A Bachelor's or graduate/higher level degree in an academic field related to the medical field, health sciences, or allied sciences, appropriate to the work of the position, is required (transcripts are required).
It is with great sadness that we inform you of the passing of Tracey Randolph on February 1, 2024 after a brief illness. Tracey joined the Office of Research Integrity (ORI) in March 2011 and was a valued member of the ORI team, serving as a Program Analyst in the Office of the Director.
In early October, ORI published a Notice of Proposed Rule Making, or NPRM. The current regulation has not been updated since 2005 and the NPRM proposed changes to the current regulation. Large numbers of researchers are subject to the rules that govern PHS-funded research—so the NPRM is an important tool to get feedback from the public and anyone involved in PHS-funded research to make their voices heard.
ORI’s Division of Education and Integrity will be hiring Health Scientist Administrators (Research Integrity Specialists) at the GS 11-12 level (pay range is $82,764 - $128,956). A Bachelor's or graduate/higher level degree in an academic field related to the medical field, health sciences, or allied sciences, appropriate to the work of the position, is required (transcripts are required).

To read the proposed updates, click here.
After more than 36 years of government service, Dr. Wanda K. Jones is retiring effective January 13, 2024. Her career is defined by a dedication to service through safeguarding public health and advocating for the people HHS serves.

Happy Holidays from the Office of Research Integrity! Back in October, ORI released a Notice of Proposed Rulemaking (NPRM). This online document proposes change to the PHS Research-Misconduct regulation, which was last updated in 2005.
Great news! You have more time to provide your input and feedback on the Notice of Proposed Rulemaking!
In the video, ORI Director Sheila Garrity discusses details about the 30-day extension. Comments on the NPRM are now due on or before Thursday, January 4, 2024.
View the video: https://youtu.be/pmFhJt5KJ-o and submit your comments: https://www.regulations.gov/document/HHS-OASH-2023-0014-0001.
ORI released a video in which ORI Director Sheila Garrity discusses why your written comments are critical for revising the current regulation. ORI is inviting all comments – no comment is too minor. View the video here: https://youtu.be/ed0bzyvrH9k
ORI released a video in which ORI Director Sheila Garrity asks for comments and feedback on the proposed changes to the regulation and discusses how feedback will be used. See the video here: https://youtu.be/jvc6RDg27pM?si=JCLh9vV5eq4uYyfU
- BLOG
Dear Colleagues,
Because ORI is in the process of revising the rule guiding its work, we want to share with you how this rulemaking process works. ORI is undertaking notice-and-comment rulemaking (also known as informal rulemaking) to update 42 CFR Part 93. Such informal rulemaking is set out in the Administrative Procedure Act (APA) and for those of you so inclined, you can read about it here:
ORI has released a new video on YouTube about the NPRM and submitting comments. The video contains detailed instructions to assist users who would like to leave a comment by December 5, 2023. View the new video here: https://youtu.be/pVC2RdYE-Q4
Submit a comment here: https://www.regulations.gov/document/HHS-OASH-2023-0014-0001
I assumed the directorship of the Office of Research Integrity (ORI) March 29, 2023, after spending over 30 years working in academia. I took on the role of research integrity officer (RIO) at Johns Hopkins University School of Medicine in 2005, the same year the current regulation took effect.
ORI has released a new video on YouTube where ORI Director Sheila Garrity discusses the NPRM and how to leave your comments. See the video here: https://youtu.be/n0VjX-8hipk?si=PaYm2727DvIg1h3j.
Use the link in the video description or go directly to the NPRM and leave a comment here: https://www.regulations.gov/document/HHS-OASH-2023-0014-0001
Dear Colleagues,
The current PHS Policy on Research Misconduct, familiarly known as “ORI’s Reg”, was brand new when I first became a research integrity officer (RIO) in the summer of 2005. The 2005 regulation greatly improved the previous, two-page regulation and provided clarity on the proper handling of research misconduct allegations. I worked with my employer, Johns Hopkins University, the leading recipient of federal funds for research at that time, to revise its policies to be in compliance with the new ORI regulation.ORI is pleased to announce the launch of its new YouTube channel! ORI’s channel will include a variety of research integrity video resources all in one place. Visit ORI’s new channel here and subscribe to receive updates about new content! View ORI’s brand new animated video about Data Management and Data Loss. This video is part of a series of three videos which will be released over the next few weeks.
On September 1, 2022, the Office of Research Integrity (ORI) published a Request for Information (RFI) in the Federal Register. ORI sought input and perspectives from individuals, research funding agencies, institutional officials, organizations, institutions, and other members of the public on the 2005 Public Health Service Policies on Research Misconduct, 42 C.F.R. Part 93, to help structure ORI’s future plans to revise the regulation. The comment period closed October 31, 2022, at 5:00 p.m. ET.

Project Title: The Future of Research IntegrityGrantee: University of South Alabama
ORI’s Division of Education and Integrity will be hiring Health Scientist Administrators (Research Integrity Specialists) at the GS 11-13 level (pay range is $78,592 - $145,617). A Bachelor's or graduate/higher level degree in an academic field related to the medical field, health sciences, or allied sciences, appropriate to the work of the position, is required (transcripts are required).
Ensuring Research Integrity - Conferences
The Office of Research Integrity (ORI) announces the anticipated availability of funds for Fiscal Year (FY) 2024 grant awards to support projects to plan and implement conferences or workshops related to ensuring research integrity and compliance with 42 C.F.R. Part 93.
OASH has posted an Expression of Interest for a Research Integrity Specialist. This is not an official job opening. To express interest in the position or learn more information, see the LinkedIn post here: Office of the Assistant Secretary for Health hiring Research Integrity Specialist (Expression of Interest) in Rockville, Maryland, United States | LinkedIn
1. Ensuring Research Integrity - Research, Development, and Demonstration
ORI's Ensuring Research Integrity - Research, Development, and Demonstration Grant Program supports projects that undertake the development and implementation of innovative practical approaches, tools, and/or resources that improve practices related to research integrity and compliance with 42 C.F.R. Part 93.
ORI has released its Annual Report for FY 2022. The report highlights ORI’s activities from the past fiscal year in the following areas:
The Office of Research Integrity (ORI) within the Office of the Assistant Secretary for Health (OASH), Department of Health and Human Services (DHHS), is seeking highly qualified candidates for two positions: a Scientist-Investigator position within the Division of Investigative Oversight and an Education and Integrity Specialist position within the Division of Education and Integrity. The positions are full-time and are located at the Rockville, MD office.
Sheila Garrity, JD, MPH, MBA has been named as the new Director of the Office of Research Integrity. She is expected to start the week of March 26, 2023.
On September 1, 2022, the Office of Research Integrity (ORI) published a Request for Information (RFI) in the Federal Register. ORI sought input and perspectives from individuals, research funding agencies, institutional officials, organizations, institutions, and other members of the general public on the 2005 Public Health Service Policies on Research Misconduct, 42 C.F.R. Part 93, to help structure ORI’s future plans to revise the regulation. The comment period closed October 31, 2022, at 5:00 p.m. ET.
The Office of Research Integrity (ORI) announces the opportunity for non-federal public and private sector entities to co-sponsor ORI conferences, workshops, symposia, meetings, roundtables, or other such events (collectively, “Events”). ORI co-sponsors a limited number of events with non-federal entities each year. Potential co-sponsors must have demonstrated interest and experience in the responsible conduct of research or handling allegations of research misconduct.
The Department of Health and Human Services (HHS), Office of Research Integrity (ORI) seeks the perspectives of individuals, research funding agencies, institutional officials, organizations, institutions, and other members of the general public on the 2005 Public Health Service Policies on Research Misconduct, 42 C.F.R. Part 93, to help structure ORI’s future plans to revise the regulation.
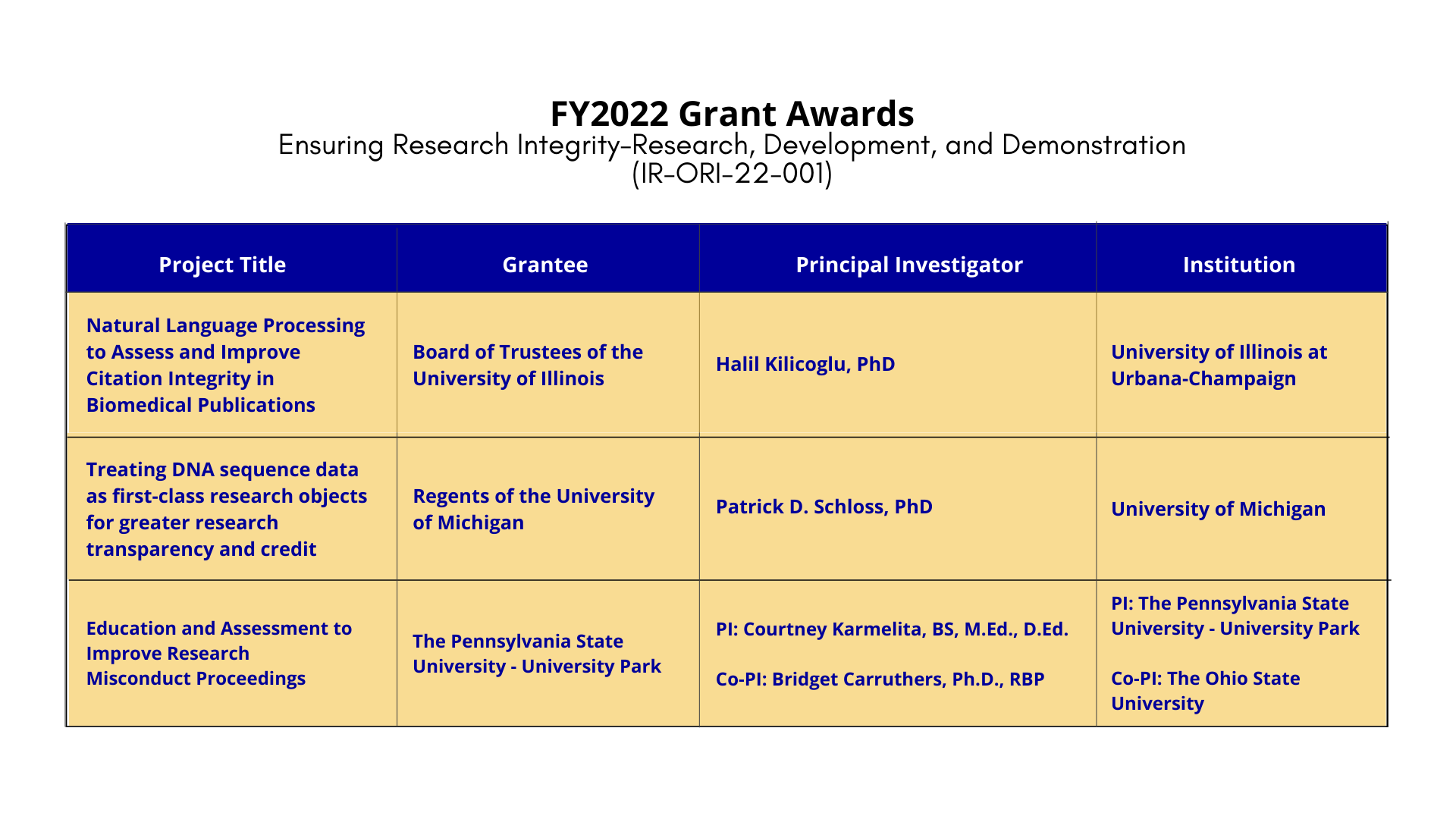
Project Title: Natural Language Processing to Assess and Improve Citation Integrity in Biomedica
Director, Office of Research Integrity
The Department of Health and Human Services,
Office of the Assistant Secretary for Health (OASH),
Office of Research Integrity (ORI)
Rockville, MDORI has released its Annual Report for FY 2021. The report highlights ORI’s activities from the past fiscal year in the following areas:
- Investigative Oversight
- Communications with Stakeholders
- Intramural Projects
- ORI's Grants Program
- ORI’s Compliance Programs
The report is available in digital (PDF) format and can be downloaded by clicking on the file below.
- BLOG
In calendar year 2021 (CY21), ORI closed 93 cases. This includes 3 cases with research misconduct findings, 26 cases in which ORI declined to pursue (DTP), 12 cases without findings of research misconduct (no-misconduct), and 52 cases closed during the assessment or inquiry stage (accession closure).
Recordings and slide decks from January 18, 2022 Notice of Funding Opportunity Technical Assistance Webinars are now available.
The Office of Research Integrity (ORI) within the Office of the Assistant Secretary for Health (OASH), Department of Health and Human Services (DHHS), is seeking highly qualified candidates for two positions: a Scientist-Investigator position within the Division of Investigative Oversight and an Education and Integrity Specialist position within the Division of Education and Integrity. Candidates interested in applying for both positions should include “Investigator/Specialist” in the subject line of their email and address this in their expression of interest.
Summary Table of FY2021 ORI Grants
ORI’s Division of Investigative Oversight (https://ori.hhs.gov/division-investigative-oversight) will be hiring a Health Scientist at the GS 13 level (pay range is $103,690 - $134,798). A Bachelor's or graduate/higher level degree in an academic field related to the medical field, health sciences, or allied sciences is required (transcripts are required at the time of application). The ideal candidate would possess a Ph.D.
Elisabeth (Lis) Handley, the former Director of ORI, has accepted a new role as the Principal Deputy Assistant Secretary for Health (PDASH) in the Office of the Assistant Secretary for Health (OASH). Dr. Wanda Jones will continue to lead ORI by serving as the Acting Director. Dr. Jones has been with ORI since December 2017, first as the Interim Director, then serving as the permanent Deputy Director since June 2019.
The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services acknowledges that an important issue in the course of institutional investigations into allegations of research misconduct is the determination of the scope. The scope of research misconduct refers to an institution diligently pursuing all significant leads discovered to be relevant to an investigation
Recordings and slide decks from the May 6, 2021 and May 10, 2021 Notice of Funding Opportunity Technical Assistance Webinars are now available.
The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services has launched the ORI File Transfer System (ORI-FTS), a FedRAMP authorized, cloud-based system for secure file transfer.
ORI has released two notice of funding opportunity announcements (NOFOs) for projects that advance the field of research integrity.
FY 2021 Notice of Funding Opportunity Announcements:
- BLOG
The Office of Research Integrity (ORI) within the Office of the Assistant Secretary for Health (OASH), Department of Health and Human Services (DHHS) is seeking a highly qualified candidate to manage activities related to assigned cases of alleged research misconduct in biomedical research studies. This position will be filled through an OASH Staff Fellow appointment (starting at a GS-11 level equivalent and up to a GS-13 level equivalent with a doctoral degree and relevant experience).
The ORI FY 2020 Annual Report has been revised to address a question raised by a reader about the way in which retractions and corrections were described in two titles on page 7. The header “Outcomes of Retractions and Corrections Requested by ORI” has been changed to “Correcting the Scientific Record.” The title of the chart, “Outcomes from Retractions/Corrections Recommended by ORI (2015-2019), n = 164” has been changed to “Status of Publications Found to Contain Research Misconduct (n=164) in ORI Findings of Research Misconduct (2015-2019).” The titles are now more in l
ORI has released its Annual Report for FY 2020. The report highlights ORI’s activities from the past fiscal year in the following areas:
ORI’s Division of Investigative Oversight will be hiring a Health Scientist at the GS 13 level (pay range is $103,690 - $134,798). A Bachelor's or graduate/higher level degree in an academic field related to the medical field, health sciences, or allied sciences is required (transcripts are required at the time of application). The ideal candidate would possess a Ph.D.
The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services acknowledges that the COVID-19 pandemic is causing disruption to the businesses and research institutions that receive funding from the Public Health Service (PHS). During this period, ORI continues to perform mission critical functions and operations with ORI staff exercising maximum telework flexibilities.
- BLOG
The Office of Research Integrity (ORI) within the Office of the Assistant Secretary for Health (OASH), Department of Health and Human Services (DHHS), is undertaking a multi-year project to study outcomes associated with retractions and corrections requested in conjunction with ORI findings of research misconduct. This project focuses on the impacts of research misconduct, found in scientific literature, on Public Health Service (PHS) funded research and human health.
ORI’s Division of Education and Integrity will be hiring a Health Science Compliance Specialist at the GS 13 level (pay range is $102,663 - $133,465). A Bachelor's or graduate/higher level degree in an academic field related to the medical field, health sciences, or allied sciences is required (transcripts are required).
ORI’s Division of Investigative Oversight will be hiring a Health Scientist at the GS 13 level (pay range is $102,663 - $133,465). A Bachelor's or graduate/higher level degree in an academic field related to the medical field, health sciences, or allied sciences is required (transcripts are required at the time of application), and the ideal candidate would possess a Ph.D or equivalent doctoral degree.
The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services invites comments on activities that foster research integrity and promote the responsible conduct of research under 42 C.F.R. Part 93. In particular, ORI is interested in learning about best practices, challenges, and needs related to teaching the responsible conduct of research, promoting research integrity, and preventing research misconduct.
Summary Table of FY2020 ORI Grants
Today on the occasion of her retirement, we have the opportunity to celebrate the many years of outstanding investigatory work that Ann Hohmann did in the service of her country, public health, and the Office of Research Integrity. Ann brought incredible tenacity, a willingness to teach others and to share her knowledge, strategic understanding of the importance of working with others like our Office of General Counsel and RIOs on the successful outcome of cases, and a keen and strong intellect to our work.
New Funding Opportunities and Technical Assistance Webinars
The Office of Research Integrity released three funding opportunities announcements for the FY2020 grant programs. Applications are due on August 4, 2020. Projects are expected to commence by September 30, 2020.
The dates and times of technical assistance webinars are listed below the corresponding funding opportunity announcements. All webinars will be recorded and posted shortly after completion.
The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services released a request for information (RFI), https://go.usa.gov/xvyHX, to invite comments on the sequestration of digital evidence during a research misconduct proceeding.

Karen Wehner, PhD Karen Wehner, Ph.D., has joined ORI as the new Director of the Division of Education and Integrity (DEI).
- BLOG
The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services acknowledges that the COVID-19 pandemic is interrupting and delaying regular operations at institutions, including research misconduct (RM) proceedings.
The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services acknowledges that the COVID-19 pandemic is causing disruption to the businesses and research institutions that receive funding from the Public Health Services (PHS). During this period, ORI continues to perform mission critical functions and operations with ORI staff working with maximum telework flexibilities. ORI is doing everything possible to accommodate the needs of the research community.
The Office of the Assistant Secretary for Health (OASH) has permanently appointed Elisabeth (Lis) Handley as the Director of ORI and Dr. Wanda Jones as the Associate Director, Research and Scientific Integrity in addition to her Deputy Director role in the office.
ORI awarded eight Research on Research Integrity projects through the Extramural Research Program, funding a total of $1,135,316. The RRI grants allows up to $150,000 in direct and indirect funding for projects that foster innovative approaches for research on research integrity and preventing misconduct.
ORI awarded five Research Integrity Conferences (RIC) grants through the Extramural Research Program, funding a total of $244,796.
----------------------------------------
NOTICE: Page updated on 02/18/2020 to reflect changes in the forecast
----------------------------------------
ORI released forecasts for three funding opportunity announcements (FOAs) for research integrity grants, including a new Program Development and Evaluation (PDE) program. ORI will continue to support the Research on Research Integrity (RRI) and the Research Integrity Conferences (RIC) grant programs.
Exploratory Workshop: Privacy and Health Research in a Data-Driven World
The HHS Office for Human Research Protections (OHRP) is hosting this full-day event on September 19, 2019. This educational event will be webcast live for easy viewing with no registration required and is free of charge! Recorded videos will also be available online after the event.
Alexander Runko, Ph.D., starting Aug. 4, will be the new Director of ORI’s Division of Investigative Oversight (DIO).
Adm. Brett P. Giroir, M.D., HHS Assistant Secretary for Health, announced today that Elisabeth Handley will join the Office of Research Integrity as the Interim Director, teaming with Deputy Director Dr. Wanda Jones, beginning August 26. She brings diverse leadership experience from within HHS. In the integrity arena, Ms. Handley was the Deputy Center Director and Acting Director of the Center for Program Integrity at the Centers for Medicare and Medicaid Services (CMS).
---------------------------------------------
UPDATE 08/26/2019: We are out of DVDs for "The Lab" and "The Research Clinic" and currently have no plans to print more copies. Both videos are available for free online.---------------------------------------------
Dr. Brett P. Giroir, the Assistant Secretary for Health, made the following announcement today:
Things to Think About: Things to Think About is a new section of the ORI blog where members of the ORI staff communicate about ideas we have about the responsible conduct of research.
Things to Think About: Things to Think About is a new section of the ORI blog where members of the ORI staff communicate about ideas we have about the responsible conduct of research.
42 C.F.R. 93.108 provides in pertinent part, “Disclosure of the identity of respondent and complainants in research misconduct proceedings is limited, to the extent possible, to those who need to know, consistent with a thorough, competent, objective and fair research misconduct proceeding, and as allowed by law.” NIH has issued a policy explaining when NIH has a need to know about matters related to research misconduct proceedings. Institutions may consider that NIH policy when determining disclosures to those who need to know, consistent with 42 C.F.R.
The Office of Research Integrity (ORI) has named Brian Mozer, PhD, to serve as the Acting Director of the Division of Investigative Oversight (DIO) on a 120-day detail appointment. Dr. Mozer joined ORI in 2012 as a contractor, becoming a federal employee in 2014. He replaces Ranjini Ambalavanar, PhD, who successfully completed her 120-day detail appointment.
Things to Think About: Things to Think About is a new section of the ORI blog where members of the ORI staff communicate about ideas we have about the responsible conduct of research.
Communicating Expectations to Your Lab Group
It is important to foster integrity in the research community. Knowledge that emerges from research is collective and rooted in core values of integrity such as accountability, honesty, fairness, objectivity and openness. Everyone involved in research should exemplify these values throughout the research process, so researchers must understand the dynamics that support or pervert practices that typify integrity in research.
About Research Participation
This is a collection of videos, infographics, and tools to inform the general public about research and research participation.
The Office of Research Integrity (ORI) has named Dr. Ranjini Ambalavanar to serve as the Acting Director of the Division of Investigative Oversight (DIO) on a 120-day detail appointment. Since joining ORI in 2009, Dr. Ambalavanar has handled more than 100 allegations of research misconduct. She replaces Dr. Ann Hohmann, who successfully completed her 120-day detail appointment.
Message from the Office of the Director
The Office of Research Integrity (ORI) works closely with Research Integrity Officers (RIOs) at institutions handling allegations of research misconduct involving biomedical or behavioral research or research training supported by the Public Health Service (PHS). Likewise, ORI provides training and educational materials for institutional Responsible Conduct of Research (RCR) Coordinators who play a role in assuring that their research institution fosters a research environment that promotes the responsible conduct of research and discourages misconduct.
COMMUNITY NEWS
New Association Provides Resources for Research Integrity Officer
Interview with Co-Founder, Lauran QualkenbushORI’s extramural program, “Research on Research Integrity” (RRI), funds empirical research that examines societal, organizational, group, and individual factors that affect research integrity. The program also funds conferences that stimulate multi-disciplinary approaches to promoting research integrity and leads to evidence-based research on research integrity.
- Quest for Research Excellence ConferenceLocation: The George Washington University, Washington, DCDate: August 7-9, 2017The Office of Research Integrity, The George Washington University (GWU), and Public Responsibility in Medicine and Research are pleased to co-sponsor the 2017 Quest for Research Excellence Conference.
- The ORI Division of Education and Integrity (DEI) has released a new series of video case studies to address integrity issues faced by those involved in the research endeavor.The videos follow the story of Dr. Jeff Thompson and his budding research lab. Amit, a postdoctoral fellow, and Ashley, a graduate student, are navigating challenges inherent to working in a competitive research environment.
- The ORI Division of Education and Integrity (DEI) has released 12 new infographics on topics related to the responsible conduct of research (RCR) and the handling of research misconduct.These infographics are designed to help young as well as experienced researchers to identify with their role in detecting and preventing research misconduct and promoting a culture of research integrity. They offer specific tips around delicate issues, such as knowing when, and how, to report questionable activity.
ORI awarded ten grant applications through Research on Research Integrity program. Five Conference grants and three new Phase I Research grants were awarded. Two successful Phase I Research projects from fiscal year 2015 were approved for Phase II funding.
Research Grants: The purpose of the Phase I research grants is to foster innovative approaches to empirical research on societal, organizational, group, and individual factors that affect, both positively and negatively, integrity in research. These grants are awarded in two phases:
- BLOGJoin the Office for Human Research Protections' (OHRP) staff at one of their upcoming events in October. Please share with your colleagues!Research Community ForumJoin OHRP staff at a Research Community Forum on October 25-26, 2016 at the Hartford Marriott Downtown Hotel in Hartford, CT.
Responsible conduct of research stems from more than one individual’s behavior - it is a learned and shared culture of promoting integrity. One of the first places new scientists are exposed to this culture is in graduate school, where they start to learn the practices and norms of their chosen field. Those who train students and post-docs have a significant influence on trainees’ perceptions of good research practices.
- The article below is from the ORI Newsletter (June 2016, v23, no2).Do you have thoughts on this topic?
- Promoting the Responsible Conduct of Research for College and University LeadersLocation: Los Angeles, CADate: Thursday, April 14, 2016 to Friday, April 15, 2016This inaugural meeting will bring together representatives from NIH, NSF, OLAW, OHRP and ORI with senior institutional officials and Research Integrity Officers to engage in discussion and develop consensus around promoting research integrity at the highest institutional level.
- Reimbursable Detail Opportunity – Health Science Administrator, HHS/Office of the Assistant Secretary for Health (OASH), Office of the Research Integrity, (ORI) – NTE 120 days. The position is located in Rockville, Maryland.Commissioned Corps O-6 or GS-0601-14. This is NOT a career-ladder opportunity.INTRODUCTIONThe ORI is seeking an individual to serve as a Health Science Administrator.
Location: San Diego, California, USA
Venue: San Diego Hilton in Harbor Island
Date: February 24-26, 2016The Office of Research Integrity (ORI) announces the appointment of a new Director, Kathryn M. Partin, PhD.
The U.S. Department of Health and Human Services (HHS) and 15 other Federal departments and agencies have announced proposed changes to The Federal Policy for the Protection of Human Subjects, also known as the “Common Rule.” The changes in the Notice of Proposed Rulemaking (NPRM) will modernize and strengthen the policy to enhance protections for research subjects.
The following case study was taken from ORI Introduction to the Responsible Conduct of Research, written by Nicholas H. Steneck. What would you do? Post a comment below.
What Should Dr. José Do?
ORI is pleased to have awarded two research grants and five conference grants through our Research on Research Integrity program.
Research Grants: The purpose of the Phase I research grants is to foster innovative approaches to empirical research on societal, organizational, group, and individual factors that affect, both positively and negatively, integrity in research. These grants are awarded in two phases:
The Office of Human Research Protections (OHRP) announces two upcoming educational opportunities on human research protections.
OHRP Research Community Forum
June 18-19, 2015
Chevy Chase, MD
Rio de Janeiro, Brazil
May 31 – June 3, 2015We are delighted to assist with the promotion of a one-day educational workshop on human research protections jointly organized by the HHS Office for Human Research Protections (OHRP) and the Office of Extramural Research (OER) of the National Institutes of Health (NIH) entitled
“Getting through Human Research Reviews without Pain”
Wednesday May 6, 2015, 9 a.m. to 4 p.m.
Renaissance Baltimore Harborplace Hotel
202 East Pratt Street, Baltimore, Maryland 21202Hank Green of CrashCourse explains the scientific method, case studies, naturalistic observation, surveys and interviews, experimentation, and proper research practices in under 11 minutes.
The Office of Research Integrity currently has two funding opportunity announcements (FOA) for research and conferences related to research integrity. Below are answers to frequently asked questions.
Where can I view the FOAs?
The FOAs can be viewed at Grants.gov
VACANCY ANNOUNCEMENT
DIRECTOR, OFFICE OF RESEARCH INTEGRITY
Department of Health and Human Services, Office of the Secretary
U.S. Office of Research Integrity Training Session
4th World Conference on Research Integrity, Rio de Janeiro, Brazil: Sunday, May 31, 2015HANDLING RESEARCH MISCONDUCT ALLEGATIONS IN A GLOBAL CONTEXT
February 26, 2015
In an effort to expand research integrity internationally, the U.S. Office of Research Integrity (ORI) is coordinating the “Research Integrity in Asia and the Pacific Rim Conference,” which will take place in Fall 2015.
The following was published in ORI's November 2014 Newsletter.
-------------------------------------------------------------------------------------
Research Integrity Officer Boot Camps
Washington, D.C., co-sponsored by the University of Virginia
March 29 – April 1, 2015
New York City, co-sponsored by the Icahn School of Medicine at Mount Sinai
April 12 – 15, 2015 The Office of Research Integrity (ORI) welcomes Zoë Hammatt. Ms.
The Office of Research Integrity (ORI) welcomes Zoë Hammatt. Ms.- The American Association for the Advancement of Science (AAAS) and the University of Pittsburgh are holding a conference for institutional officials to train them to implement research ethics training.
Below is an update on potential grant applicants for ORI′s Funding Opportunity Announcement (https://www.grants.gov/view-opportunity.html?oppId=248593) for research on the Responsible Conduct of Research and Research Integrity.
____________________________________________
Salary Limitation Update:
Update (03/31/2014)
The full version of The Research Clinic is now available at
https://ori.hhs.gov/TheResearchClinicBelow are questions from potential grant applicants for ORI′s Funding Opportunity Announcement (https://www.grants.gov/view-opportunity.html?oppId=248593) for research on the Responsible Conduct of Research and Research Integrity.
Question: ″What is the budget limitation in the FOA?″
Answer: The budget limitation is $150,000 per year, including direct and indirect costs. The second year awards are conditional depending on funding availability.
The Annual Report on Possible Research Misconduct (ARPRM, Form PHS-6349) was updated on December 31, 2013. The new form now requires institutions that receive PHS funding to include the contact information for the Research Integrity Officer (RIO) and the Responsible Conduct of Research (RCR) Coordinator.
- The Funding Opportunity Announcement (FOA) for research on research integrity grants was published on December 2, 2013. ORI intends to support up to four awards with a maximum budget of $150,000 per year. Second-year funding may be available depending on successful completion of the first year and available funds in FY 2014.
- ORI will soon release a series of RCR case studies edited by Dr. James Dubois of St. Louis Univerity. The creation of the case studies was funded through ORI's RCR Resource Development program and involved a team of nearly 20 writers, contributors, and reviewers. These well-crafted case studies, along with role playing scenarios, will be available for instructors to incorporate into their institutions' RCR training programs.
"The Lab: Avoiding Research Misconduct" is now available with Spanish subtitles. This version greatly increases the reach of The Lab to Spanish-speaking researchers and research administrators nationally and internationally. Spanish is among the top three languages spoken in the world. This translation allows "The Lab" to be accessible to thousands of Spanish-speaking researchers and students who may one day come to the US to further their training.
On April 18, 2013, the Office of Research Integrity (ORI) published a notice of 2 findings of research misconduct by Dr. Bois in the Federal Register at 78 Fed. Reg. 23255 (.pdf).
Dr. James DuBois, St. Louis University, was awarded a contract from the ORI RCR Resource Development Program to create an RCR casebook with case studies and role playing activities. ORI will be releasing the finished casebook shortly via the ORI website.
Below is case study from the Authorship and Publications chapter of the book.
My Lab Boss Puts His Name on My Papers and Proposals
Denise McCartney speaks about conflict of interest and research integrity. McCartney is the Associate Vice Chancellor for Research Administration at WUSTL.
Space is filling up fast so don't forget to register for the "ORI at 20: Reassessing Research Integrity" conference. This year's meeting will provide rich content on handling allegations of research misconduct and fostering an environment for responsible research. Attendees will leave the conference with the skills and knowledge to better perform their roles as part of research community.
The constant attention to retractions brought forward by RetractionWatch.com along with a steady flow of news regarding research fraud has brought the topic of retractions to the mainstream amongst scientists. Seemingly, more researchers are reading published articles with higher scrutiny not only as learners, but as reviewers.
The Office of Research Integrity (ORI) was formed in 1992 with the merger of the NIH Office of Scientific Integrity (OSI) and the Office of Scientific Integrity Review (OSIR) within the Office of the Assistant Secretary for Health, HHS. Twenty years later, ORI has evolved into an office that goes beyond investigating allegations of research misconduct--ORI has become a partner with the research community to help improve the quality of research and improve the public trust in research.
When the Office of Research Integrity (ORI) and the European Science Foundation (ESF) held the First World Conference on Research Integrity in 2007, we had no idea what would become of it. Held in Lisbon, the meeting attracted hundreds of participants from nearly 50 countries.
In 2010, ORI screened The Lab at the 2nd World Conference on Research Integrity in Singapore.
In February 2011, the Office of Research Integrity (ORI) launched “The Lab,” an interactive training video on the handling and prevention of research misconduct in laboratories. Each week, hundreds of learners watch The Lab, which is available for free on the ORI website. With the success of this program, ORI has joined forces with the Office of Human Research Protections (OHRP) to pursue the development of a second training product. This time, the focus is on clinical research and social science research.
The topic of plagiarism seems to have gained popularity in the recent past. More and more companies are offering online plagiarism detection services, and several universities are adopting these services to catch academic dishonesty.
The RePAIR Program provides a formal method for rehabilitating researchers who have engaged in research misconduct or questionable research practices. This novel concept has raised an important questions. Why should researchers who engage in wrongdoing be allowed to be remediated? Do they deserve a second chance? Is it fair to other researchers that diligently seek funding?
The Office of Research Integrity (ORI) and Johns Hopkins Medicine are co-sponsoring a conference which will be held in Baltimore, MD on April 3-5, 2013. Entitled "Reassessing Research Integrity," the meeting will take a look at the past, present, and future of research integrity. Participants will be challenged to examine and evaluate the effectiveness of responsible conduct of research (RCR) training and explore what the future holds for RCR.
RePAIR (Restoring Professionalism and Integrity in Research) is a new program that “provides intensive professional development education for investigators who have engaged in wrongdoing or unprofessional behavior, including persistent non-compliance,” according to its leaders. RePAIR was developed with NIH funding by a team of clinical, organizational, and developmental psychologists with input from remediation trainers, ethicists, lawyers, investigators and research administrators.
The question of what researchers can learn from violations of integrity lies at the center of MindOpen’s summer issue (https://www.mindopen.nl/pdf/mindopen_number_9_summer_2012.pdf). The digizine swirls around a case of large-scale fraud committed by social scientist Diederik Stapel who was outed for fabricating data and suspended from Tilburg University last fall.
Dr. John Galland retired last week as the Director of Division of Education and Integrity. Dr. Galland joined ORI in March 2009, bringing a unique approach to research education. During his tenure at ORI, he was involved with several activities to promote the Responsible Conduct of Research and prevent research misconduct. Before joining ORI, he directed the U.C. Davis Laboratory Management Institute which incorporated an innovative method for training scientists on administering a successful lab environment. ORI wishes Dr. Galland well on his future endeavors.
Can science journalists do more to uncover research misconduct?
No clear consensus emerged from the gathering of 250 science journalists and publishing officials at the Royal Society in London. In fact, the panel discussion and internet chatter probably raised more questions than they answered:
Social scientist Leon Festinger did some fascinating studies in the 1950s on “cognitive dissonance.” He used the term to describe the uncomfortable tension people feel when they find their actions are inconsistent with their beliefs. Rather than change their behavior to fit their beliefs, they will change what they believe to be true to alleviate the tension.
- BLOG
ORI expanded its social media presence when it joined Twitter.com one year ago on July 6, 2011. In the past year, we've used Twitter to inform the research community about conferences, research misconduct findings, education resources for research, and other research-oriented news. We don't just use Twitter to post news--We use Twitter to engage in two-way communication with the community. If you're not already doing so, we encourage you to join the other 350+ ORI followers who benefit from our social media campaign.
Here are some recent tweets:
Yesterday, a group comprising of government departments, research funders, and other stakeholders in the UK released a document that states their support to promote research integrity. The "Concordat" outlines responsibilities and expectations for researchers, research employers, and funders in regards to promoting rigorous research and the handling of research misconduct.
The following commitments to research are introduced:
Responsible Conduct of Research (RCR) training has been around in some format (9 core areas and now 10 or more) for over a decade. After several years, many have wondered if RCR training has done what it was intended to do: prevent research misconduct and promote integrity. In the July 2012 issue of Academic Medicine (https://www.ncbi.nlm.nih.gov/pubmed/22622208), Dr. Donald Kornfeld’s assessment is that the surveys done to date have not demonstrated that training has changed behavior.
No matter how different the words are that you're using, when you are paraphrasing other people's works, it's always a good idea to provide the source of your information. Miguel Roig, from St. John's University, lays out 24 guidelines for preventing plagiarism. Number 5 on the list stresses the importance of citing paraphrased materials.
Providing proper citation may seem to be an easy chore compared to bench science, yet, this simple practice can be a source of problems if done incorrectly. Miguel Roig, from St. John's University, lays out 24 guidelines for preventing plagiarism. Number 14 on the list stresses the importance of proper citation.
A researcher, speaking at a conference, presents two sets of results based on two related datasets. He references a paper that he published recently, describing in great detail the first set of results based on the first dataset. This paper mentions the second dataset but does not discuss the results based on it. A colleague attending the conference asks for a copy of both datasets.
Your government-funded research at a university lab has produced results, published in a peer-reviewed journal, that have great potential for commercial application. A for-profit corporation requests a copy of your data, saying that they want this for use in their own research, and they have no intention of commercializing your results. You strongly believe that the potential for commercial application is compelling and unavoidable. How should you proceed?
Romantic relationships have developed among doctoral candidates working in a large university laboratory on several occasions. Invariably these relationships have led to bad feelings, either among those involved or among their colleagues in the lab, and productivity has suffered as a result. The director seeks to establish a policy that would sustain morale in such cases, preferably by requiring that at least one party to any such relationship must leave the lab. Is this appropriate? How should the director proceed?
Held on March 15-16, 2012 in Washington DC, the Quest for Research Excellence 2012 conference provided an outstanding means to enhance the professional development and behavior of researchers:
“What areas do you think research integrity hasn't explored enough?” This question prompted different answers during the Quest for Research Excellence 2012 Conference being held in Georgetown, March 15-16. Here are some areas in research integrity that a few people attending the conference and giving presentations would like to see further explored:
“It is an extraordinary privilege to be a scientist,” says Dr. Marlene Belfort in her keynote address at the Quest for Research Excellence 2012 Conference being held in Georgetown, March 15-16. There have been a number of points raised in discussions, presentations, and posters ranging from the role institutions play in promoting research integrity to the role the media play in publicizing what has been done in research. The following are a few research challenges raised in talks and discussions.
The Mini RIO Boot Camp at the Quest for Research Excellence Conference, which required pre-registration, is full! Those interested in knowing when the next full-scale RIO boot camp will be scheduled, should email their contact information to AskORI@hhs.gov
Marlene Belfort is a Distinguished Professor at SUNY Albany. She will be speaking about Work-Life balance for researchers at the ORI Quest for Research Excellence 2012 Conference. She will present "Work-Life Balance, Domestic Equilibrium and Research Excellence" on Thursday, March 15th at 8am.
BIO
The "Quest for Research Excellence" 2012 conference will take place March 15-16, 2012, and will be at Georgetown University's Leavey Conference Center and Hotel, 3800 Reservoir Road, NW, Washington DC 20057.
Plenary speakers include:
Plagiarism is an issue that has plagued mankind for centuries. Along with falsification and fabrication of data, the act of plagiarism constitutes research misconduct as defined by ORI. Although it's one of the three misconduct behaviors, it has historicially made up a small percentage of research misconduct findings by ORI, until recently.
ORI started the new year by launching the new website to better serve the research community. The new site replaces the old ORI site which was originally designed over 10 years ago. The completely revamped site is Section 508 compliant , user-friendly, and allows visitor to get to almost all content within one or two clicks. The new website is built on Drupal, the same open-source Content Management System (CMS) used by WhiteHouse.gov and many other popular sites.
We are pleased to announce that Dr. David E. Wright will be joining ORI in January as the new Director.
From 1993-2004, Dr. Wright served as Michigan State University’s (MSU) Assistant Vice President for Research Ethics and Standards, as well as its Intellectual Integrity Officer, overseeing most of MSU’s research regulatory compliance activity. He has also chaired the University’s Committee on Research Involving Human Subjects for 11 years.





