Module 3: Elements of Research - Section 6
Section 6: Blinding
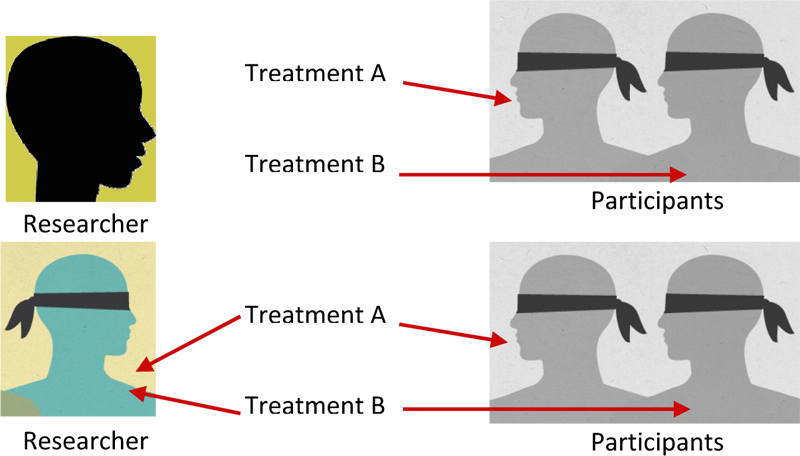 Blinding is a technique used to decrease bias on the part of the researcher or the participant. In some studies, the participant is not told to which group they have been assigned. This is called single blinding. There is another level of blinding called double blinding where neither the researcher nor the participant know which group the participant is in until this information is revealed at the end of the study. Blinding can reduce the temptation to ignore random assignment procedures and can reduce any expectations about the potential effectiveness of the treatment or program since group assignment remains unknown by the participant, the researcher or both the participant and researcher. The results are more likely to provide information about the true effect of the treatment or program being tested when blinding is used.
Blinding is a technique used to decrease bias on the part of the researcher or the participant. In some studies, the participant is not told to which group they have been assigned. This is called single blinding. There is another level of blinding called double blinding where neither the researcher nor the participant know which group the participant is in until this information is revealed at the end of the study. Blinding can reduce the temptation to ignore random assignment procedures and can reduce any expectations about the potential effectiveness of the treatment or program since group assignment remains unknown by the participant, the researcher or both the participant and researcher. The results are more likely to provide information about the true effect of the treatment or program being tested when blinding is used.
Definition: Blinding is a technique used to decrease bias on the part of the researcher or the participant where the participant, or both the researcher and the participant are not told to which group they have been assigned.
Case Example for Blinding
In a study investigating the effects of a new anti-itch cream, participants with minor skin rashes were randomly divided into one of three groups. In the first group, participants received a new anti-itch cream that contained 3% hydrocortisone. In the second group, the participants received the standard cream available over-the-counter which contains 1% hydrocortisone. The third and final group members received a cream that contained 0% hydrocortisone. The participants did not know to which group they were assigned. Participants used the cream for three days to relieve itching symptoms caused by the rash. After three days, each participant’s rash was examined to determine if itching symptoms subsided. If this was not the case, the participant was provided with the standard treatment, which is known to relieve itching.
Section 6: Discussion Questions
- Why are participants not told (blind to) their group assignment?
- Is this a single or double blind study?
- Do you think knowledge of group assignment (no blinding) would affect the results? Why or why not?
- Do you think that the researcher’s knowledge of group assignment would affect the results? Why or why not?





