Module 3: Elements of Research - Section 5
Section 5: Random Assignment
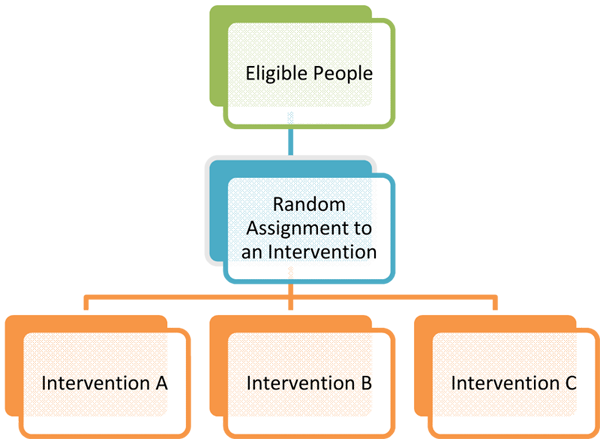
 Random assignment is a procedure used in experiments to create multiple study groups that include participants with similar characteristics so that the groups are equivalent at the beginning of the study. The procedure involves assigning individuals to an experimental treatment or program at random, or by chance (like the flip of a coin). This means that each individual has an equal chance of being assigned to either group. Usually in studies that involve random assignment, participants will receive a new treatment or program, will receive nothing at all or will receive an existing treatment. When using random assignment, neither the researcher nor the participant can choose the group to which the participant is assigned.
Random assignment is a procedure used in experiments to create multiple study groups that include participants with similar characteristics so that the groups are equivalent at the beginning of the study. The procedure involves assigning individuals to an experimental treatment or program at random, or by chance (like the flip of a coin). This means that each individual has an equal chance of being assigned to either group. Usually in studies that involve random assignment, participants will receive a new treatment or program, will receive nothing at all or will receive an existing treatment. When using random assignment, neither the researcher nor the participant can choose the group to which the participant is assigned.
The benefit of using random assignment is that it "evens the playing field." This means that the groups will differ only in the program or treatment to which they are assigned. If both groups are equivalent except for the program or treatment that they receive, then any change that is observed after comparing information collected about individuals at the beginning of the study and again at the end of the study can be attributed to the program or treatment. This way, the researcher has more confidence that any changes that might have occurred are due to the treatment under study and not to the characteristics of the group.
 A potential problem with random assignment is the temptation to ignore the random assignment procedures. For example, it may be tempting to assign an overweight participant to the treatment group that includes participation in a weight-loss program. Ignoring random assignment procedures in this study limits the ability to determine whether or not the weight loss program is effective because the groups will not be randomized. Research staff must follow random assignment protocol, if that is part of the study design, to maintain the integrity of the research. Failure to follow procedures used for random assignment prevents the study outcomes from being meaningful and applicable to the groups represented.
A potential problem with random assignment is the temptation to ignore the random assignment procedures. For example, it may be tempting to assign an overweight participant to the treatment group that includes participation in a weight-loss program. Ignoring random assignment procedures in this study limits the ability to determine whether or not the weight loss program is effective because the groups will not be randomized. Research staff must follow random assignment protocol, if that is part of the study design, to maintain the integrity of the research. Failure to follow procedures used for random assignment prevents the study outcomes from being meaningful and applicable to the groups represented.
Definition: Random assignment is a procedure used in experiments to create study groups with similar characteristics so that the groups are equivalent at the beginning of the study.
Case Example for Random Assignment
In a study to help individuals quit smoking, investigators randomly assigned participants to one of two groups. In Group A, participants took a class to quit smoking. The classes took place each week for 10-weeks and included information about the benefits of quitting smoking. In addition, participants in the class received strong social support from mentors or "buddies." In the Group B, participants read a 3-page pamphlet created by the American Cancer Association that explains the benefits of quitting smoking. The investigator randomly assigned participants to one of the two groups. It was found that those who participated in the class and received support from their buddies were more likely to quit smoking compared to those in the other group that received only the pamphlet.





