ORI Case Closures
In calendar year 2021 (CY21), ORI closed 93 cases. This includes 3 cases with research misconduct findings, 26 cases in which ORI declined to pursue (DTP), 12 cases without findings of research misconduct (no-misconduct), and 52 cases closed during the assessment or inquiry stage (accession closure). The table below illustrates how this compares with ORI case closures over the past 15 years.
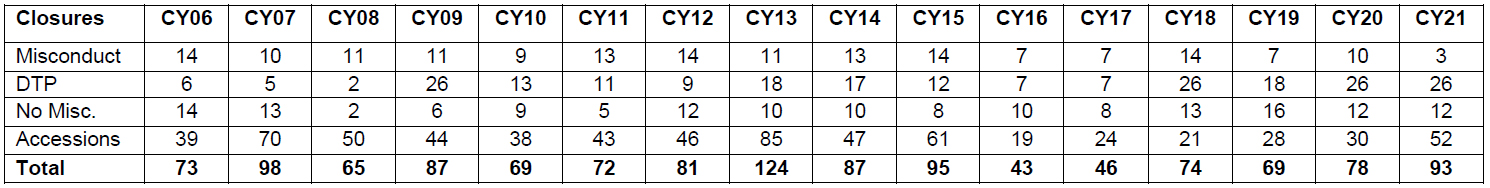
As noted in the table above, ORI cases can be closed in several ways – misconduct, DTP, no-misconduct, or accession closure. Misconduct, DTP, and no-misconduct closures generally involve cases in which the institution conducted an investigation into an allegation(s) of research misconduct1 and ORI subsequently conducted an oversight review of that investigation.
For a misconduct closure, based on a preponderance of the evidence during its oversight review, ORI found that a respondent committed research misconduct for one or more allegations involving Public Health Service (PHS) funded research. Consequently, HHS makes a finding of research misconduct against the respondent and imposes administrative actions, which may include debarment, supervision, certification, prohibition from PHS advisory service, retraction and/or correction of the affected scientific literature, and/or other administrative actions as described in 42 C.F.R. § 93.407.
A DTP closure involves a case in which an institution found that research misconduct occurred and may implement administrative actions against the respondent, but during its oversight review, ORI determined that a separate PHS finding of research misconduct was not warranted. Generally, ORI makes this determination based on a variety of factors, such as evidentiary weaknesses of the case, lack of ORI jurisdiction over the allegation(s) for which the institution made a finding(s), or lack of significance of the misconduct. ORI’s DTP closure of a case is not an exoneration of the respondent and does not diminish the authority of the institution to draw its own conclusion about scientific or professional misconduct by the respondent.
A no-misconduct closure involves a case in which the institution conducted an investigation and determined that research misconduct did not occur. Based on a preponderance of the evidence during its oversight review, ORI concurred with the institution’s determination.
An accession closure involves a case that was resolved during the assessment or inquiry stage of the institutional proceeding. Generally, during its assessment or inquiry into the allegation(s), the institution determined that there was insufficient evidence to warrant proceeding to an investigation. Based on its subsequent review of the institution’s assessment or inquiry report, ORI concurred with the institution. Accession closures also can involve matters in which the institution concluded that proceeding to investigation was warranted, but ORI determined that the allegation(s) fall outside of ORI’s jurisdiction in accordance with 42 C.F.R. Part 93.
1In instances in which a respondent admits to research misconduct, the case may be closed prior to the completion of an investigation. See 42 C.F.R. § 93.316.
